What Is The Electron Configuration For Potassium
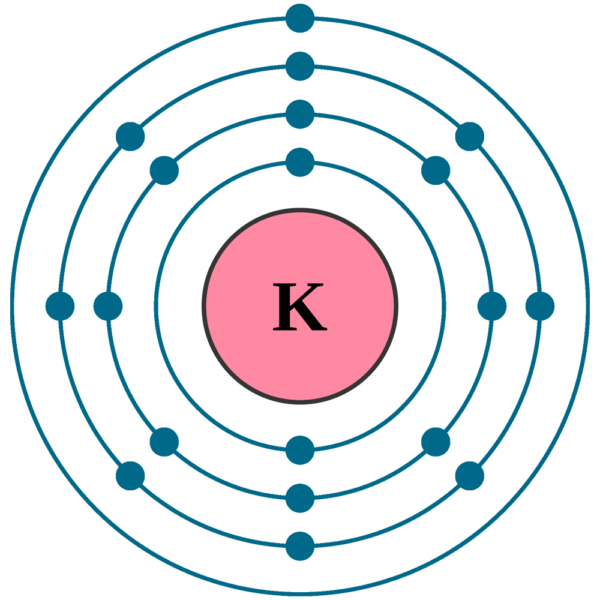
Potassium, with the atomic number 19, is an alkali metal found in the first group of the periodic table. Understanding its electron configuration is crucial for grasping its chemical behavior and reactivity. Let’s break down the electron configuration of potassium step by step, exploring its significance and how it aligns with the principles of quantum mechanics.
The Basics of Electron Configuration
Electron configuration describes the arrangement of electrons in an atom’s energy levels, or shells, and their respective subshells (s, p, d, f). The configuration is determined by the Aufbau principle, Pauli exclusion principle, and Hund’s rule, which govern how electrons fill orbitals.
Potassium’s Electron Configuration
Potassium has 19 electrons. To determine its electron configuration, we follow the order of filling orbitals based on increasing energy levels:
- 1s²: The first two electrons fill the 1s orbital.
- 2s²: The next two electrons fill the 2s orbital.
- 2p⁶: The next six electrons fill the 2p orbitals.
- 3s²: The next two electrons fill the 3s orbital.
- 3p⁶: The next six electrons fill the 3p orbitals.
- 4s¹: The final electron occupies the 4s orbital.
Thus, the complete electron configuration of potassium is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹.
This can also be represented using the noble gas shorthand notation:
[Ar] 4s¹, where [Ar] represents the electron configuration of argon (the nearest noble gas with atomic number 18).
Why Potassium’s Configuration Matters
Potassium’s electron configuration highlights its position in the periodic table and its chemical properties:
- Alkali Metal: As a Group 1 element, potassium readily loses its single valence electron to achieve a stable, noble gas configuration (like argon).
- Reactivity: The low ionization energy of the 4s electron makes potassium highly reactive, especially with electronegative elements like oxygen and halogens.
- Role in Biology: Potassium ions (K⁺) are essential for nerve function, muscle contraction, and maintaining osmotic balance in living organisms.
Visualizing Potassium’s Electron Configuration
Imagine a series of concentric shells around the nucleus. The innermost shell (1s) holds 2 electrons, the next shell (2s and 2p) holds 8 electrons, the third shell (3s and 3p) holds another 8 electrons, and the outermost shell (4s) holds the single valence electron. This structure explains why potassium is soft, has a low melting point, and is highly reactive.
Practical Applications of Potassium’s Configuration
Understanding potassium’s electron configuration is vital in various fields:
- Chemistry: Predicts its behavior in reactions, such as forming ionic compounds like potassium chloride (KCl).
- Biology: Explains the role of K⁺ ions in cellular processes.
- Industry: Potassium is used in fertilizers, soaps, and as a heat exchange medium in nuclear reactors.
Comparative Analysis: Potassium vs. Other Alkali Metals
Potassium shares similarities with other Group 1 elements (e.g., sodium, lithium) due to their common electron configuration pattern (ns¹). However, potassium’s larger atomic size and lower ionization energy make it more reactive than lithium but less so than cesium.
| Element | Atomic Number | Electron Configuration | Reactivity |
|---|---|---|---|
| Lithium (Li) | 3 | 1s² 2s¹ | Moderate |
| Sodium (Na) | 11 | 1s² 2s² 2p⁶ 3s¹ | High |
| Potassium (K) | 19 | 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ | Very High |
Future Implications: Potassium in Emerging Technologies
Potassium’s unique electron configuration makes it a candidate for innovative applications:
- Batteries: Potassium-ion batteries are being explored as a cheaper alternative to lithium-ion batteries.
- Nuclear Energy: Potassium compounds are used in heat transfer fluids for nuclear reactors.
- Agriculture: Sustainable farming practices rely on potassium-rich fertilizers to enhance crop yields.
FAQ Section
Why does potassium have a +1 charge in compounds?
+Potassium loses its single valence electron (4s¹) to achieve a stable electron configuration, forming a K⁺ ion with a +1 charge.
How does potassium’s electron configuration differ from sodium?
+Sodium has one less electron (11) and its configuration is 1s² 2s² 2p⁶ 3s¹, while potassium has 19 electrons with a configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹.
Can potassium form compounds with a charge other than +1?
+Rarely. Potassium’s strong tendency to lose its single valence electron makes +1 its most stable oxidation state.
Why is potassium reactive with water?
+Potassium’s low ionization energy allows it to easily lose its 4s¹ electron, reacting vigorously with water to form potassium hydroxide (KOH) and hydrogen gas (H₂).
How does potassium’s configuration relate to its biological importance?
+The ease of losing its valence electron allows potassium to form K⁺ ions, which are crucial for nerve impulse transmission and maintaining cellular osmotic balance.
Conclusion
Potassium’s electron configuration, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹, is the foundation of its chemical and physical properties. From its reactivity in the lab to its essential role in life processes, understanding this configuration provides insights into its behavior across diverse applications. As technology advances, potassium’s unique characteristics will continue to make it a valuable element in both traditional and emerging fields.

