Name For Hbro4

Introduction to HBrO4: Unraveling the Chemistry Behind the Compound
In the realm of chemistry, every compound has a unique story to tell, and HBrO4 is no exception. This perbromic acid, a powerful oxidizing agent, plays a significant role in various chemical processes. To truly understand HBrO4, we must delve into its nomenclature, properties, and applications, uncovering the intricacies that make it a fascinating subject of study.
The Nomenclature of HBrO4: A Systematic Approach
The name “HBrO4” might seem like a random assortment of letters and numbers, but it follows a systematic naming convention established by the International Union of Pure and Applied Chemistry (IUPAC). According to IUPAC guidelines, the name of a compound is derived from its constituent elements and their oxidation states.
In the case of HBrO4: - H represents hydrogen, with an oxidation state of +1 - Br represents bromine, with an oxidation state of +7 (the highest possible for bromine) - O represents oxygen, with an oxidation state of -2
Using the IUPAC nomenclature rules, we can break down the name as follows: - The prefix “per-” indicates the highest oxidation state of the central atom (bromine) - The suffix “-ic” denotes the presence of oxygen in the compound - The prefix “brom-” refers to the bromine atom - The suffix “-ate” is used for compounds containing oxygen
Combining these elements, we arrive at the systematic name: Perbromic Acid.
Alternative Names and Common Usage
While “Perbromic Acid” is the IUPAC-approved name, HBrO4 is also known by other names, including: - Bromic(VII) Acid: Emphasizing the oxidation state of bromine - Perbromate(V) Acid: Highlighting the perbromate ion (BrO4-)
In laboratory settings and chemical literature, the abbreviation “HBrO4” is widely used due to its simplicity and convenience.
Chemical Properties and Structure
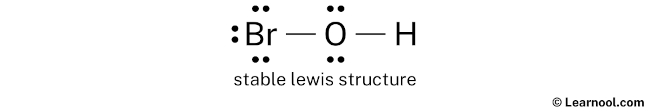
HBrO4 is a strong acid, readily dissociating into hydrogen ions (H+) and perbromate ions (BrO4-) in aqueous solution. Its molecular structure consists of a central bromine atom bonded to four oxygen atoms, with one additional oxygen atom bonded to a hydrogen atom.
Applications and Uses
HBrO4 finds applications in various fields, including: - Analytical Chemistry: As a reagent for determining the concentration of reducing agents - Organic Synthesis: As an oxidizing agent in the synthesis of complex organic compounds - Water Treatment: As a disinfectant and oxidizing agent for removing impurities
Safety Considerations
Handling HBrO4 requires caution due to its corrosive and oxidizing nature. Proper personal protective equipment (PPE), including gloves, goggles, and lab coats, should be worn when working with this compound.
Comparative Analysis: HBrO4 vs. Other Oxidizing Agents
To better understand the unique properties of HBrO4, let’s compare it with other common oxidizing agents:
tr>| Compound | Oxidizing Power | Stability | Applications |
|---|---|---|---|
| HBrO4 | Very High | Moderate | Analytical Chemistry, Organic Synthesis |
| HClO4 | High | High | Inorganic Synthesis, Electrochemistry |
| HNO3 | Moderate | High | Nitration Reactions, Explosives Manufacturing |
Historical Context and Discovery
The discovery of HBrO4 dates back to the late 19th century, when chemists were exploring the properties of bromine and its compounds. Early researchers, including German chemist Johannes Thiele, played a pivotal role in characterizing the properties of perbromic acid and its salts.
Future Research and Developments
As our understanding of HBrO4 continues to evolve, researchers are exploring new applications and synthetic routes for this compound. Recent studies have focused on: - Green Chemistry: Developing environmentally friendly methods for synthesizing HBrO4 - Materials Science: Investigating the use of HBrO4 in the synthesis of advanced materials - Biomedical Applications: Exploring the potential of HBrO4 as an antimicrobial agent
Key Takeaways
Frequently Asked Questions (FAQs)
What is the chemical formula for Perbromic Acid?
+The chemical formula for Perbromic Acid is HBrO4, consisting of hydrogen (H), bromine (Br), and oxygen (O) atoms.
How does HBrO4 compare to other oxidizing agents in terms of strength?
+HBrO4 is one of the strongest oxidizing agents, surpassing compounds like HNO3 and rivaling HClO4 in terms of oxidizing power.
What precautions should be taken when handling HBrO4?
+When handling HBrO4, wear appropriate personal protective equipment (PPE), including gloves, goggles, and lab coats, and work in a well-ventilated area to minimize exposure risks.
Can HBrO4 be used in organic synthesis?
+Yes, HBrO4 is used as an oxidizing agent in organic synthesis, particularly for the oxidation of alcohols and other functional groups.
What is the role of HBrO4 in analytical chemistry?
+In analytical chemistry, HBrO4 is used as a reagent for determining the concentration of reducing agents through redox titrations and other analytical techniques.
Conclusion: Unlocking the Potential of HBrO4
In conclusion, HBrO4 is a fascinating compound with a rich history and a wide range of applications. From its systematic nomenclature to its powerful oxidizing properties, this perbromic acid continues to captivate chemists and researchers alike. As our understanding of HBrO4 deepens, we can expect to uncover new uses and synthetic routes, further expanding its role in various fields. Whether you’re a student, researcher, or simply a chemistry enthusiast, the story of HBrO4 serves as a testament to the beauty and complexity of the chemical world.



