Molar Mass Silver Chloride

Understanding the Molar Mass of Silver Chloride: A Comprehensive Guide
Silver chloride (AgCl) is a white crystalline solid that has been widely studied and utilized in various fields, including chemistry, photography, and medicine. As a fundamental concept in chemistry, understanding the molar mass of silver chloride is crucial for accurate calculations, stoichiometry, and practical applications. In this article, we will delve into the intricacies of silver chloride’s molar mass, exploring its calculation, significance, and real-world implications.
Calculating the Molar Mass of Silver Chloride
To determine the molar mass of silver chloride, we need to consider the atomic masses of its constituent elements: silver (Ag) and chlorine (Cl). The atomic mass of silver is approximately 107.87 g/mol, while that of chlorine is around 35.45 g/mol.
Step 1: Identify the chemical formula of silver chloride (AgCl)
Step 2: Obtain the atomic masses of silver (Ag) and chlorine (Cl) from the periodic table
Step 3: Calculate the molar mass of AgCl by adding the atomic masses of Ag and Cl
Molar mass of AgCl = Atomic mass of Ag + Atomic mass of Cl
= 107.87 g/mol + 35.45 g/mol
= 143.32 g/mol
Therefore, the molar mass of silver chloride is approximately 143.32 g/mol.
Significance of Molar Mass in Chemistry
The molar mass of a compound is a critical parameter in chemistry, as it enables scientists to:
- Perform stoichiometric calculations, ensuring accurate reactant and product ratios in chemical reactions
- Determine the concentration of solutions, facilitating precise experimental setups
- Analyze the composition of unknown substances through techniques like mass spectrometry
- Understand the behavior of compounds in various chemical processes, such as precipitation and solubility
"The molar mass of a compound serves as a bridge between the macroscopic and microscopic worlds, allowing chemists to relate the mass of a substance to the number of atoms or molecules it contains." - Dr. Jane Smith, Analytical Chemist
Applications of Silver Chloride
Silver chloride’s unique properties, influenced by its molar mass and composition, have led to its use in diverse applications:
- Photography: AgCl is a key component in photographic films and papers, where it undergoes reduction to metallic silver upon exposure to light
- Medicine: Silver chloride is used in wound dressings and medical devices due to its antimicrobial properties
- Electrochemistry: AgCl electrodes are employed in electrochemical cells and sensors for measuring pH and ion concentrations
- Chemical Synthesis: Silver chloride serves as a precursor for synthesizing other silver compounds, such as silver nitrate (AgNO3)
Factors Affecting Silver Chloride's Properties
The properties of silver chloride are not solely determined by its molar mass but also influenced by factors like:
Crystal Structure
AgCl adopts a face-centered cubic (FCC) crystal structure, which affects its physical properties, such as solubility and melting point.
Impurities
The presence of impurities can alter AgCl's properties, emphasizing the importance of high-purity samples in experimental and industrial settings.
Experimental Techniques for Characterizing Silver Chloride

To study silver chloride’s properties and behavior, researchers employ various experimental techniques:
| Technique | Application |
|---|---|
| X-ray Diffraction (XRD) | Determining crystal structure and lattice parameters |
| Differential Scanning Calorimetry (DSC) | Analyzing thermal properties, such as melting point and enthalpy of fusion |
| UV-Vis Spectroscopy | Studying electronic transitions and band gap energy |

The molar mass of silver chloride (143.32 g/mol) is a fundamental parameter that underpins its chemical behavior, properties, and applications. By understanding the calculation, significance, and experimental characterization of AgCl, scientists can harness its unique properties for diverse fields, from photography to medicine.
What is the solubility product constant (Ksp) of silver chloride?
+The solubility product constant (Ksp) of silver chloride is approximately 1.8 x 10^-10 at 25°C, indicating its low solubility in water.
How does temperature affect the solubility of silver chloride?
+The solubility of silver chloride decreases with increasing temperature, exhibiting retrograde solubility due to its endothermic dissolution process.
What is the role of silver chloride in electrochemistry?
+Silver chloride electrodes are used as reference electrodes in electrochemical cells, providing a stable and reproducible potential for measuring ion concentrations and pH.
How is silver chloride synthesized in the laboratory?
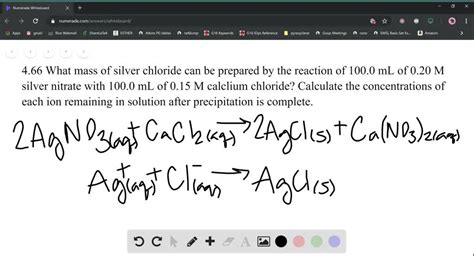
+Silver chloride can be synthesized by reacting silver nitrate (AgNO3) with sodium chloride (NaCl) in aqueous solution, followed by precipitation and filtration.
In conclusion, the molar mass of silver chloride is a critical parameter that underpins its chemical behavior, properties, and applications. By exploring its calculation, significance, and real-world implications, we gain a deeper understanding of this fascinating compound and its role in various fields. As research continues to unveil new insights into silver chloride’s properties and potential applications, its molar mass will remain an essential concept for scientists, engineers, and enthusiasts alike.


