Liquid Dissolved In Liquid
The Fascinating World of Liquid-Liquid Solutions: A Deep Dive into Molecular Interactions
Liquids dissolving in liquids is a fundamental process that underpins everything from industrial chemical reactions to the biological functions within our cells. At its core, this phenomenon is governed by the intricate dance of molecules, where one liquid (the solute) disperses uniformly into another (the solvent). This article explores the science behind liquid-liquid dissolution, its real-world applications, and the factors that influence its behavior.
The Molecular Ballet: How Liquids Dissolve in Liquids
Dissolution occurs when the intermolecular forces between solute and solvent molecules are stronger than those within the solute or solvent alone. This process is driven by entropy—the tendency of systems to move toward disorder. When a solute dissolves, its molecules spread out, increasing randomness and lowering the overall free energy of the system.
Factors Influencing Liquid-Liquid Dissolution
Molecular Polarity
Polar molecules have uneven charge distributions, creating dipole-dipole interactions. Nonpolar molecules, lacking these charges, rely on weaker London dispersion forces. This difference dictates solubility—polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes.Temperature
Heat increases molecular kinetic energy, enhancing solubility for most liquids. However, some systems exhibit retrograde solubility, where solubility decreases with temperature due to changes in molecular interactions.Pressure
Unlike gases, liquids are relatively incompressible, so pressure has minimal effect on solubility. However, in specialized cases (e.g., deep-sea chemistry), pressure can influence molecular behavior.Molecular Size and Structure
Smaller molecules generally dissolve more readily than larger ones due to greater surface area for interaction. Additionally, branched or complex molecular structures can hinder dissolution.
Real-World Applications: Where Liquid-Liquid Dissolution Shines
1. Pharmaceutical Industry
Many drugs are formulated as liquid solutions for faster absorption. For example, intravenous medications rely on the dissolution of active compounds in saline or dextrose solutions.
2. Chemical Manufacturing
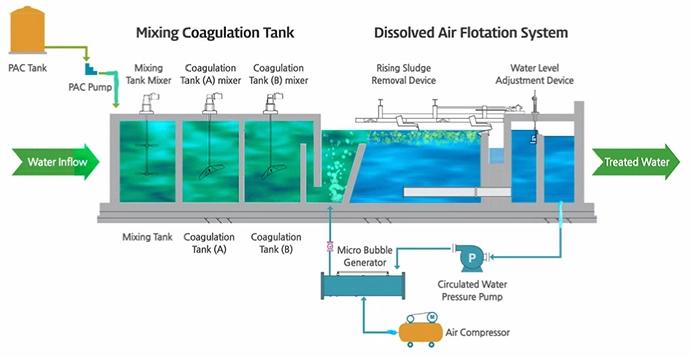
Liquid-liquid extraction is a cornerstone of chemical synthesis. For instance, separating rare earth metals involves dissolving them in organic solvents and then recovering them through precipitation.
3. Food and Beverage Industry
Flavorings, colorings, and preservatives are dissolved in liquids to create consistent products. The solubility of sugar in water is a classic example, underpinning everything from soft drinks to syrups.
4. Environmental Science
Understanding liquid-liquid dissolution is crucial for modeling pollutant behavior in water bodies. For example, oil spills involve the interaction of nonpolar hydrocarbons with polar water, influencing cleanup strategies.
Case Study: The Role of Liquid-Liquid Dissolution in Biodiesel Production
Biodiesel is produced through transesterification, where triglycerides (from vegetable oils or animal fats) react with alcohol in the presence of a catalyst. The process relies on the dissolution of alcohol (e.g., methanol) in the oil phase to facilitate the reaction. Efficient mixing and solubility are critical for yield and purity.
Future Trends: Pushing the Boundaries of Liquid-Liquid Dissolution
Advances in nanotechnology and green chemistry are expanding the possibilities of liquid-liquid dissolution. Researchers are developing novel solvents, such as ionic liquids, which offer tunable properties and reduced environmental impact. Additionally, machine learning is being used to predict solubility behavior, accelerating material discovery.
Myth vs. Reality: Common Misconceptions
| Myth | Reality |
|---|---|
| All liquids dissolve in each other. | Solubility is governed by molecular compatibility (e.g., polar vs. nonpolar). |
| Temperature always increases solubility. | Some liquids exhibit retrograde solubility, where solubility decreases with temperature. |
| Pressure significantly affects liquid solubility. | Pressure has minimal impact on most liquid-liquid systems. |
Practical Guide: Enhancing Liquid-Liquid Dissolution
FAQ Section
Why don’t oil and water mix?
+Oil is nonpolar, while water is polar. The strong hydrogen bonds in water repel nonpolar molecules, preventing dissolution.
How does temperature affect liquid solubility?
+For most liquids, solubility increases with temperature as molecules gain kinetic energy. However, some systems show retrograde solubility due to changes in intermolecular forces.
What is liquid-liquid extraction?
+Liquid-liquid extraction is a separation technique where a solute is transferred from one liquid phase to another immiscible liquid phase, often used in chemical and pharmaceutical industries.
Can nonpolar solvents dissolve polar solutes?
+Generally, no. Polar solutes require polar solvents for dissolution due to the nature of intermolecular forces.
What are ionic liquids, and why are they important?
+Ionic liquids are salts in liquid form at room temperature. They are valuable as green solvents due to their low volatility and tunable properties.
Conclusion: The Ubiquitous Nature of Liquid-Liquid Dissolution
From the laboratory to the factory floor, liquid-liquid dissolution is a silent enabler of modern technology. Its principles govern processes as diverse as drug delivery and environmental remediation, highlighting its importance across disciplines. As science advances, our ability to manipulate and harness this phenomenon will only grow, unlocking new possibilities for innovation and sustainability.
"In the world of liquids, dissolution is not just a process—it’s a gateway to transformation."
By understanding the molecular intricacies and practical applications of liquid-liquid dissolution, we can better appreciate its role in shaping our world. Whether in a test tube or a production line, this fundamental process continues to inspire and enable progress.



