Lewis Dot Diagram Of Potassium
Understanding the Lewis Dot Diagram of Potassium
Potassium (K), a soft, silvery-white alkali metal, plays a crucial role in various biological and industrial processes. Its atomic structure, particularly its electron configuration, is fundamental to understanding its chemical behavior. The Lewis dot diagram, a visual representation of an atom’s valence electrons, provides valuable insights into potassium’s reactivity and bonding patterns.
Electron Configuration and Valence Electrons
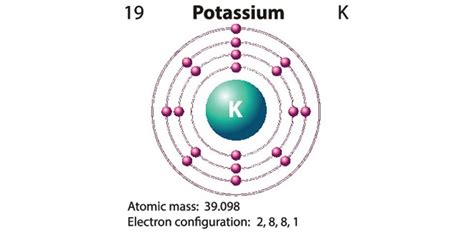
Potassium has an atomic number of 19, meaning it possesses 19 electrons. Its electron configuration is as follows:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹
The outermost shell, also known as the valence shell, contains a single electron in the 4s orbital. This lone valence electron is the key to potassium’s chemical properties.
Lewis Dot Diagram Representation
The Lewis dot diagram for potassium (K) is remarkably simple, reflecting its single valence electron:
K·
Here, the symbol ‘K’ represents the potassium atom, and the single dot (·) signifies its lone valence electron.
Significance of the Lewis Dot Diagram
- Reactivity: Potassium’s single valence electron makes it highly reactive. It readily donates this electron to achieve a stable, noble gas configuration (similar to argon, Ar). This tendency to lose an electron explains potassium’s strong metallic character and its ability to form ionic compounds, such as potassium chloride (KCl).
- Ionic Bonding: In ionic compounds, potassium typically forms a +1 ion (K⁺) by losing its single valence electron. This positively charged ion is attracted to negatively charged ions, forming a lattice structure characteristic of ionic compounds.
- Periodic Trends: Potassium’s Lewis dot diagram illustrates the periodic trend of increasing reactivity down Group 1 (alkali metals). As we move down the group, the valence electron becomes farther from the nucleus, making it easier to remove and increasing the metal’s reactivity.
Practical Applications
Understanding potassium’s Lewis dot diagram is essential in various fields:
- Biology: Potassium ions (K⁺) are vital for nerve impulse transmission, muscle contraction, and maintaining cellular fluid balance in living organisms.
- Agriculture: Potassium is a key nutrient for plant growth, playing a crucial role in photosynthesis, water regulation, and disease resistance.
- Industry: Potassium compounds are used in fertilizers, soaps, detergents, and as a heat transfer medium in nuclear reactors.
Frequently Asked Questions (FAQ)
Why does potassium have only one dot in its Lewis dot diagram?
+Potassium has only one valence electron in its outermost shell (4s¹). The single dot in its Lewis dot diagram represents this lone electron.
How does potassium's Lewis dot diagram relate to its position in the periodic table?
+Potassium's position in Group 1 (alkali metals) of the periodic table directly relates to its single valence electron. This characteristic electron configuration is consistent with other Group 1 elements, all of which have a single electron in their outermost s orbital.
What is the difference between the Lewis dot diagram of potassium and that of sodium?
+Both potassium (K) and sodium (Na) are alkali metals with a single valence electron. Therefore, their Lewis dot diagrams are identical, each represented by a single dot: Na· and K·.
Can potassium form covalent bonds?
+While potassium primarily forms ionic bonds by losing its single valence electron, it can participate in covalent bonding under specific conditions, such as in certain organometallic compounds. However, this is less common compared to its ionic bonding behavior.
How does the Lewis dot diagram help predict potassium's chemical behavior?
+The Lewis dot diagram of potassium, with its single valence electron, indicates its strong tendency to lose this electron and form a +1 ion (K⁺). This prediction aligns with potassium's observed chemical behavior, such as its reactivity with water and its ability to form ionic compounds with nonmetals.
Conclusion
The Lewis dot diagram of potassium, with its single dot representing the lone valence electron, is a concise yet powerful tool for understanding this element’s chemical nature. It highlights potassium’s reactivity, its role in ionic bonding, and its position within the periodic table. This simple diagram serves as a foundation for comprehending potassium’s diverse applications in biology, agriculture, and industry.



