Formula Of Sodium Fluoride
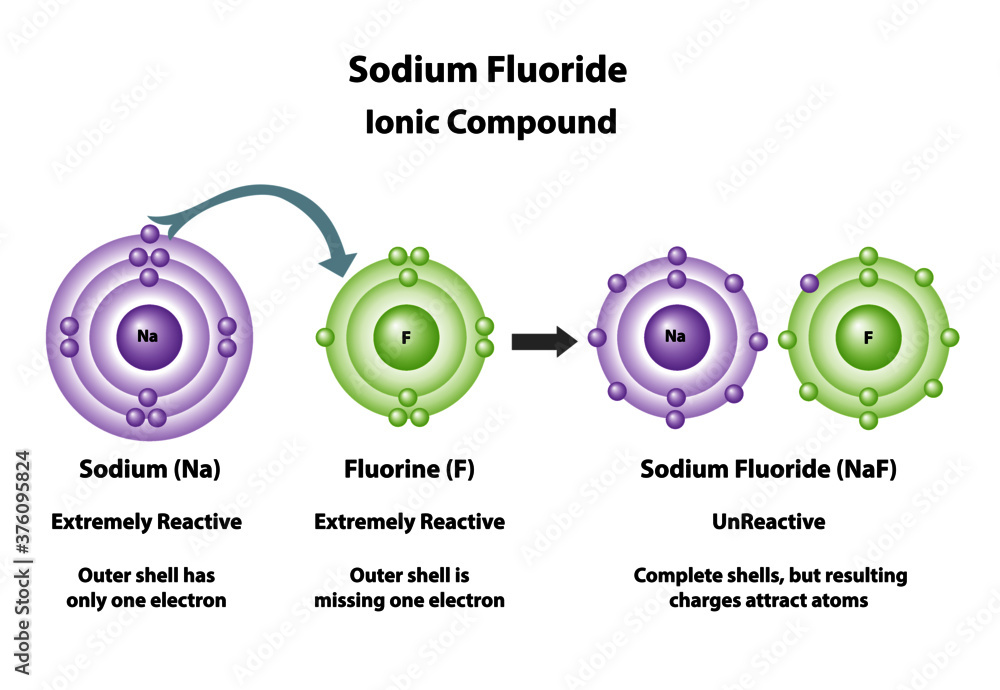
Sodium fluoride (NaF) is an inorganic compound with the chemical formula NaF. It is a simple ionic compound composed of sodium (Na⁺) and fluoride (F⁻) ions held together by strong electrostatic forces. This article delves into the properties, structure, synthesis, applications, and safety considerations of sodium fluoride, providing a comprehensive understanding of this widely used chemical.
Chemical Structure and Bonding
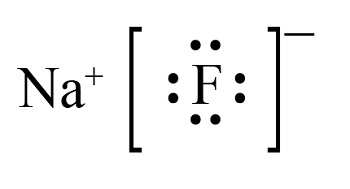
Sodium fluoride adopts a cubic crystal lattice structure known as the rock salt structure. In this arrangement:
- Sodium ions (Na⁺) occupy lattice sites, each surrounded by six fluoride ions (F⁻) in an octahedral geometry.
- Fluoride ions (F⁻) occupy the interstices, with each fluoride ion surrounded by six sodium ions.
This highly symmetric structure is a result of the strong ionic bonding between Na⁺ and F⁻, which arises from the large difference in electronegativity between sodium (0.93) and fluorine (3.98).
The rock salt structure is a common motif in ionic compounds, characterized by its high stability and efficient packing of ions. Sodium fluoride's lattice energy, a measure of the strength of ionic bonds, is approximately 923 kJ/mol, reflecting the compound's high melting point (993°C) and hardness.
Synthesis of Sodium Fluoride
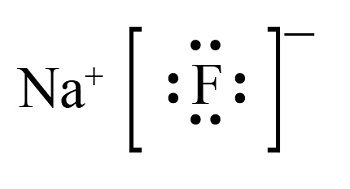
Sodium fluoride is primarily produced through the neutralization reaction of sodium hydroxide (NaOH) with hydrofluoric acid (HF):
NaOH + HF → NaF + H₂O
This reaction is highly exothermic and must be carefully controlled to prevent overheating. Alternatively, sodium fluoride can be synthesized by reacting sodium carbonate (Na₂CO₃) with hydrofluoric acid:
Na₂CO₃ + 2HF → 2NaF + H₂O + CO₂
Industrial Production Steps:
- Raw Material Preparation: Sodium hydroxide or sodium carbonate is prepared in aqueous solution, while hydrofluoric acid is carefully handled due to its corrosive nature.
- Reaction: The reactants are mixed in a controlled environment to ensure complete neutralization.
- Filtration: The resulting sodium fluoride is filtered to remove any insoluble impurities.
- Drying: The product is dried to obtain anhydrous sodium fluoride.
Physical and Chemical Properties
| Property | Value |
|---|---|
| Molecular Weight | 41.99 g/mol |
| Melting Point | 993°C (1819°F) |
| Boiling Point | 1704°C (3099°F) |
| Solubility in Water | 4.1 g/100 mL (20°C) |
| Density | 2.558 g/cm³ |

Sodium fluoride is a white, crystalline solid that is highly soluble in water, forming a colorless solution. It is chemically stable under normal conditions but reacts vigorously with strong acids, releasing toxic hydrogen fluoride (HF).
Applications of Sodium Fluoride
Sodium fluoride finds extensive use in various industries due to its unique properties:
1. Dental Health
Sodium fluoride is a key ingredient in toothpaste and mouthwash due to its ability to prevent dental caries. Fluoride ions strengthen tooth enamel by promoting the formation of fluorapatite, a more acid-resistant mineral than hydroxyapatite.2. Water Fluoridation
Many communities add sodium fluoride to drinking water to reduce the incidence of tooth decay. The optimal concentration is typically around 0.7–1.2 ppm (parts per million).3. Chemical Manufacturing
Sodium fluoride is used as a fluorinating agent in organic synthesis, particularly in the production of pharmaceuticals and agrochemicals. It is also employed in the manufacture of specialty glasses and enamels.4. Metallurgy
In metallurgy, sodium fluoride is used as a flux to lower the melting point of metals and remove impurities during welding and casting processes.5. Pest Control
Sodium fluoride is a component of rodenticides, where it acts as a toxic bait for rats and mice. Its high toxicity to mammals necessitates careful handling and regulated use.Pros and Cons of Sodium Fluoride Use
Pros: Effective in preventing tooth decay, widely available, and cost-effective for water fluoridation.
Cons: Potential health risks at high concentrations, environmental concerns related to water contamination, and ethical debates over mass medication.
Safety and Environmental Considerations

Sodium fluoride is moderately toxic and poses health risks if ingested, inhaled, or absorbed through the skin. Acute exposure can cause symptoms such as nausea, vomiting, and abdominal pain, while chronic exposure may lead to skeletal fluorosis, a condition characterized by joint pain and bone fractures.
"Fluoride’s benefits in preventing dental caries are well-established, but its safe use requires careful monitoring and regulation to avoid adverse effects." – World Health Organization (WHO)
Environmental concerns include the potential contamination of water bodies and soil, which can harm aquatic life and ecosystems. Proper disposal and handling are critical to minimize these risks.
Comparative Analysis: Sodium Fluoride vs. Other Fluorides
| Compound | Formula | Solubility in Water (g/100 mL) | Primary Use |
|---|---|---|---|
| Sodium Fluoride | NaF | 4.1 (20°C) | Dental health, water fluoridation |
| Calcium Fluoride | CaF₂ | 0.0016 (20°C) | Optics, manufacturing |
| Ammonium Fluoride | NH₄F | Soluble | Etching, chemical synthesis |
Sodium fluoride stands out for its high solubility and effectiveness in dental applications, whereas calcium fluoride is less soluble and primarily used in optics.
Future Trends and Research
Ongoing research focuses on optimizing fluoride delivery systems to maximize dental benefits while minimizing risks. Innovations include the development of controlled-release fluoride materials and targeted therapies for high-risk populations. Additionally, studies are exploring the environmental impact of fluoride compounds and sustainable methods for their production and disposal.
Sodium fluoride remains a vital compound in dental health and industrial applications, but its use must be balanced with safety and environmental considerations. Continued research and regulation are essential to harness its benefits responsibly.
What is the chemical formula of sodium fluoride?
+The chemical formula of sodium fluoride is NaF.
How does sodium fluoride prevent tooth decay?
+Sodium fluoride prevents tooth decay by promoting the formation of fluorapatite, a more acid-resistant mineral than hydroxyapatite, which strengthens tooth enamel.
Is sodium fluoride safe for consumption?
+Sodium fluoride is safe in controlled amounts, such as in toothpaste and fluoridated water. However, excessive ingestion can lead to fluoride toxicity, causing symptoms like nausea and, in severe cases, skeletal fluorosis.
What are the environmental concerns associated with sodium fluoride?
+Environmental concerns include water and soil contamination, which can harm aquatic life and ecosystems. Proper disposal and regulation are essential to mitigate these risks.
Can sodium fluoride be used in industries other than dental health?
+Yes, sodium fluoride is used in chemical manufacturing, metallurgy, pest control, and the production of specialty glasses and enamels.
In conclusion, sodium fluoride (NaF) is a versatile compound with significant applications in dental health, industry, and beyond. Its unique properties, coupled with careful regulation and research, ensure its continued relevance in addressing global health and industrial challenges.

