Endergonic Reactions Release Energy
The Misconception of Endergonic Reactions and Energy Release
In the realm of biochemistry and thermodynamics, the terms “endergonic” and “exergonic” are often used to describe the nature of chemical reactions. A common misconception is that endergonic reactions release energy, but this is fundamentally incorrect. To clarify this, let’s delve into the intricacies of these reactions, their mechanisms, and their implications in biological and chemical systems.
Understanding Endergonic Reactions
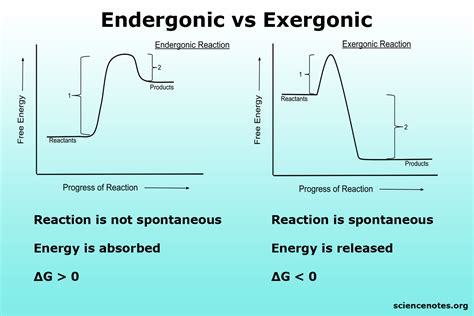
Endergonic reactions are processes that require an input of energy to proceed. The term “endergonic” originates from the Greek words “endon” (inside) and “ergon” (work), implying that work is done on the system. In these reactions, the Gibbs free energy change (ΔG) is positive (ΔG > 0), indicating that the products have higher free energy than the reactants. This means the system becomes less stable, and energy must be absorbed from the surroundings to drive the reaction forward.
Comparing Endergonic and Exergonic Reactions
To better understand endergonic reactions, it’s helpful to contrast them with exergonic reactions. Exergonic reactions release energy (ΔG < 0), making them spontaneous under standard conditions. The energy released can be in the form of heat, light, or other usable forms, such as ATP in biological systems.
| Aspect | Endergonic Reaction | Exergonic Reaction |
|---|---|---|
| Gibbs Free Energy (ΔG) | Positive (ΔG > 0) | Negative (ΔG < 0) |
| Energy Flow | Energy absorbed from surroundings | Energy released to surroundings |
| Spontaneity | Non-spontaneous | Spontaneous |
| Example | Protein synthesis | Cellular respiration |

Biological Relevance of Endergonic Reactions
In biological systems, endergonic reactions are essential for processes that require energy input, such as:
- Protein Synthesis: The assembly of amino acids into proteins is endergonic, requiring ATP to form peptide bonds.
- Active Transport: Moving molecules against their concentration gradient across cell membranes demands energy, often supplied by ATP.
- DNA Replication: The synthesis of new DNA strands from nucleotides is an endergonic process, reliant on energy from ATP and other high-energy molecules.
Mechanisms Driving Endergonic Reactions
Endergonic reactions are typically coupled with exergonic reactions to ensure their completion. This coupling is exemplified by the concept of energy coupling in biology. For instance, ATP hydrolysis (an exergonic reaction) provides the energy needed to drive endergonic reactions.
Historical Evolution of Endergonic Reaction Concepts
The understanding of endergonic reactions has evolved significantly over the centuries. Early chemists focused on heat exchange in reactions, but the advent of thermodynamics in the 19th century introduced concepts like Gibbs free energy, which provided a more comprehensive framework for analyzing reaction energetics.
Myth vs. Reality: Endergonic Reactions and Energy Release
Myth: Endergonic reactions release energy.
Reality: Endergonic reactions absorb energy from their surroundings to proceed.
This misconception likely arises from confusion with exergonic reactions or a misunderstanding of the term “endergonic.” Clarifying this distinction is crucial for accurately describing biochemical processes.
Future Trends in Endergonic Reaction Research
Advances in bioenergetics and synthetic biology are shedding new light on endergonic reactions. Researchers are exploring ways to harness endergonic processes for applications like energy storage and sustainable chemistry. For example, artificial photosynthesis aims to replicate endergonic reactions to convert sunlight into chemical energy.
Practical Application Guide
For students and researchers, understanding endergonic reactions is essential for fields like biochemistry, molecular biology, and bioengineering. Here’s a practical guide to mastering these concepts:
- Study Thermodynamics: Familiarize yourself with Gibbs free energy (ΔG) and its role in determining reaction spontaneity.
- Analyze Biological Pathways: Examine how endergonic reactions are coupled with exergonic ones in metabolic pathways.
- Experiment with Models: Use computational models to simulate endergonic reactions and observe their energy requirements.
FAQ Section
What is the primary energy source for endergonic reactions in cells?
+The primary energy source for endergonic reactions in cells is ATP (adenosine triphosphate), which is generated through exergonic processes like cellular respiration.
Can endergonic reactions occur without coupling to exergonic reactions?
+Under standard conditions, endergonic reactions cannot occur spontaneously without an external energy source, such as coupling to an exergonic reaction.
How do endergonic reactions contribute to cellular growth?
+Endergonic reactions are essential for synthesizing macromolecules like proteins and nucleic acids, which are critical for cellular growth and repair.
What role does temperature play in endergonic reactions?
+Temperature can influence the rate of endergonic reactions but does not change their non-spontaneous nature. Higher temperatures may increase reaction rates but still require an energy input.
Conclusion
Endergonic reactions are fundamental to the functioning of biological and chemical systems, despite the common misconception that they release energy. By absorbing energy, these reactions enable essential processes like protein synthesis, active transport, and DNA replication. Understanding the distinction between endergonic and exergonic reactions is crucial for advancing fields like biochemistry and bioenergetics. As research progresses, the manipulation of endergonic reactions holds promise for innovative applications in energy production and sustainable technologies.



