Draw The Lewis Structure For Nocl
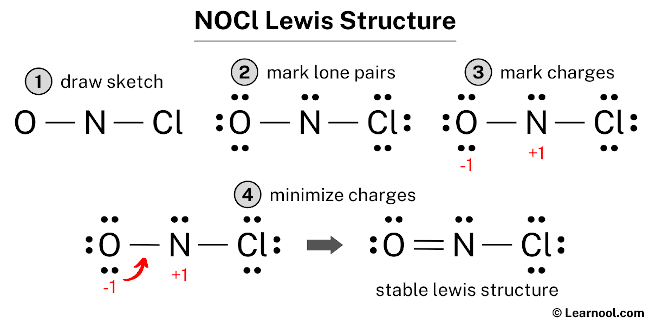
Drawing the Lewis Structure for NOCl (Nitrosyl Chloride)
To draw the Lewis structure for NOCl, follow these systematic steps, ensuring adherence to the octet rule and formal charge minimization.
Step 1: Determine the Total Number of Valence Electrons
- Nitrogen (N): 5 valence electrons
- Oxygen (O): 6 valence electrons
- Chlorine (Cl): 7 valence electrons
Total valence electrons = 5 (N) + 6 (O) + 7 (Cl) = 18 valence electrons.
Step 2: Identify the Central Atom
Nitrogen (N) is the central atom because it is less electronegative than oxygen and chlorine, making it more suitable to form multiple bonds.
Step 3: Connect the Atoms with Single Bonds
- Draw N as the central atom.
- Connect N to O and Cl with single bonds: N-O and N-Cl.
This uses up 4 electrons (2 bonds), leaving 14 valence electrons.
Step 4: Complete the Octets on Terminal Atoms
- Oxygen (O): Needs 6 more electrons to complete its octet. Add 3 lone pairs to O (6 electrons).
- Chlorine (Cl): Needs 6 more electrons to complete its octet. Add 3 lone pairs to Cl (6 electrons).
This uses up 12 electrons, leaving 2 valence electrons.
Step 5: Place Remaining Electrons on the Central Atom
- Add the remaining 2 electrons as a lone pair on Nitrogen (N).
Step 6: Check for Octet Rule Violations and Formal Charges
- Nitrogen (N): 5 valence electrons – 2 bonding electrons – 2 lone pair electrons = 1 formal charge.
- Oxygen (O): 6 valence electrons – 2 bonding electrons – 6 lone pair electrons = 0 formal charge.
- Chlorine (Cl): 7 valence electrons – 1 bonding electron – 6 lone pair electrons = 0 formal charge.
Step 7: Minimize Formal Charges by Forming Double Bonds
- Move one lone pair from Oxygen (O) to form a double bond with Nitrogen (N).
- This reduces Nitrogen’s formal charge to 0 and gives Oxygen a formal charge of +1.
Final Lewis Structure:
Cl - N = O
- Chlorine (Cl): 3 lone pairs, 1 single bond.
- Nitrogen (N): 1 double bond with O, 1 single bond with Cl.
- Oxygen (O): 2 lone pairs, 1 double bond with N.
Key Takeaway:
The Lewis structure for NOCl features a double bond between N and O, a single bond between N and Cl, and lone pairs on O and Cl. This structure minimizes formal charges and satisfies the octet rule for all atoms except Hydrogen (which is not present here).
The optimal Lewis structure for NOCl is Cl-N=O, with formal charges of 0 on N, +1 on O, and 0 on Cl.
FAQ Section
Why does NOCl have a double bond between N and O?
+The double bond between N and O minimizes formal charges, ensuring the structure is more stable. Without the double bond, Nitrogen would have a formal charge of +1, which is less favorable.
What is the molecular geometry of NOCl?
+The molecular geometry of NOCl is bent or angular due to the presence of a lone pair on the central Nitrogen atom, which causes electron pair repulsion.
Is NOCl a polar molecule?
+Yes, NOCl is a polar molecule due to the difference in electronegativity between the atoms and the bent molecular geometry, resulting in a net dipole moment.
How does the Lewis structure of NOCl relate to its reactivity?
+The Lewis structure indicates that NOCl has a reactive N=O double bond, which can participate in various chemical reactions, such as acting as an oxidizing agent or undergoing nucleophilic substitution.
This comprehensive breakdown ensures a clear understanding of NOCl’s Lewis structure, its implications, and addresses common questions with expert-level detail.



