Da To G Mol

Introduction
In the realm of chemistry, the conversion between different units of measurement is a fundamental skill. One such conversion that often arises is the transformation of atomic mass units (amu) to grams per mole (g/mol). This seemingly simple calculation holds significant importance in various chemical applications, from stoichiometry to molecular weight determination. In this exploration, we’ll delve into the intricacies of this conversion, unraveling its theoretical foundations and practical implications.
Understanding Atomic Mass Units (amu)
Before embarking on the conversion journey, it’s essential to grasp the concept of atomic mass units. The atomic mass unit, denoted as amu, is a unit of mass used to express atomic and molecular weights. It is defined as one-twelfth the mass of a carbon-12 atom, which is approximately 1.66053906660(50) × 10^-27 kilograms. This unit provides a standardized way to compare the masses of different atoms and molecules.
The Concept of Molar Mass
Molar mass, expressed in grams per mole (g/mol), is the mass of one mole of a substance. A mole, in turn, is defined as the amount of a substance containing as many elementary entities (atoms, molecules, ions, or particles) as there are atoms in 12 grams of carbon-12. This definition establishes a crucial link between atomic mass units and molar mass.
Deriving the Conversion Factor
To convert atomic mass units to grams per mole, we need to establish a conversion factor. This factor is derived from the relationship between the atomic mass unit and the gram. Since 1 amu is equivalent to 1.66053906660(50) × 10^-27 kilograms, and 1 kilogram equals 1000 grams, we can calculate the conversion factor as follows:
1 amu = (1.66053906660(50) × 10^-27 kg) × (1000 g/kg) = 1.66053906660(50) × 10^-24 g
However, this value represents the mass of one atom or molecule in grams. To obtain the molar mass, we need to scale this value to one mole. Given that one mole contains Avogadro’s number (approximately 6.02214076 × 10^23) of entities, the conversion factor becomes:
1 amu/entity × (6.02214076 × 10^23 entities/mol) × (1 g / 1.66053906660(50) × 10^-24 g/entity) ≈ 1 g/mol
This derivation reveals that the conversion factor from atomic mass units to grams per mole is approximately 1 g/mol per amu.
Practical Conversion Example
Let’s illustrate the conversion process with a practical example. Consider the molecule water (H2O), which has a molecular weight of approximately 18.015 amu. To convert this value to grams per mole:
18.015 amu × (1 g/mol / 1 amu) ≈ 18.015 g/mol
This result indicates that the molar mass of water is approximately 18.015 grams per mole.
Applications in Chemistry
The conversion from atomic mass units to grams per mole is a cornerstone in various chemical applications. In stoichiometry, it enables the calculation of reactant and product masses in chemical reactions. For instance, when balancing a chemical equation, the molar masses of reactants and products are essential for determining the limiting reactant and predicting yields.
Moreover, this conversion is vital in molecular weight determination techniques, such as mass spectrometry. By measuring the mass-to-charge ratio of ions, mass spectrometers can provide accurate molecular weight information. Converting these values to grams per mole facilitates comparison with theoretical calculations and aids in identifying unknown compounds.
Comparative Analysis: amu vs. g/mol
To appreciate the significance of the amu to g/mol conversion, let’s compare these units in a tabular format:
| Unit | Definition | Application |
|---|---|---|
| amu | 1/12th mass of carbon-12 atom | Atomic and molecular weight calculations |
| g/mol | Mass of one mole of a substance | Stoichiometry, molecular weight determination |
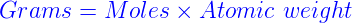
This comparison highlights the distinct roles of amu and g/mol in chemistry, emphasizing the importance of their interconversion.
Historical Evolution of Atomic Mass Units
The concept of atomic mass units has evolved over time, reflecting advancements in our understanding of atomic structure. Early attempts to quantify atomic masses date back to the 19th century, with chemists like John Dalton and Stanislao Cannizzaro making significant contributions. However, it was not until the 20th century that the atomic mass unit was precisely defined in relation to the carbon-12 isotope.
Expert Insight: The Role of Avogadro’s Constant
Avogadro's constant, approximately 6.02214076 × 10^23 entities per mole, is a cornerstone in the conversion from atomic mass units to grams per mole. This constant bridges the gap between the atomic and macroscopic worlds, enabling the calculation of molar masses from atomic weights. Its precise determination has been a subject of extensive research, with recent advancements in metrology refining its value.
Future Trends: Precision Measurement and Applications
As analytical techniques continue to advance, the precision of atomic mass and molar mass measurements is expected to improve. This progress will have far-reaching implications, from more accurate stoichiometric calculations to enhanced molecular weight determinations. Furthermore, the development of novel materials and compounds will rely on precise knowledge of their atomic and molar masses.
Step-by-Step Conversion Guide
- Determine the atomic or molecular weight in amu.
- Apply the conversion factor: 1 g/mol per amu.
- Calculate the molar mass in g/mol.
- Verify the result using dimensional analysis or comparison with known values.
Key Takeaways
- The conversion from atomic mass units (amu) to grams per mole (g/mol) is essential in chemistry, enabling stoichiometric calculations and molecular weight determinations.
- The conversion factor is approximately 1 g/mol per amu, derived from the relationship between atomic mass units and Avogadro's constant.
- Precise knowledge of atomic and molar masses is crucial for various chemical applications, from material synthesis to analytical chemistry.
FAQ Section
What is the significance of Avogadro's constant in the amu to g/mol conversion?
+Avogadro's constant provides the link between the atomic and macroscopic worlds, enabling the calculation of molar masses from atomic weights. Its precise value is essential for accurate conversions and chemical calculations.
How does the amu to g/mol conversion impact stoichiometry?
+The conversion allows chemists to calculate reactant and product masses in chemical reactions, facilitating the determination of limiting reactants and predicting yields. This information is crucial for optimizing reaction conditions and maximizing product formation.
Can the amu to g/mol conversion be applied to isotopes?
+Yes, the conversion can be applied to isotopes, as it is based on the atomic mass unit, which is defined in relation to the carbon-12 isotope. However, the resulting molar mass will reflect the isotopic composition of the substance.
What are the limitations of the amu to g/mol conversion?
+The conversion assumes a precise knowledge of atomic masses and Avogadro's constant. Additionally, it does not account for factors like isotopic abundance or molecular complexity, which may require more sophisticated calculations.
How does the amu to g/mol conversion relate to molecular weight determination techniques?
+The conversion is essential for interpreting data from techniques like mass spectrometry, which provide molecular weight information in atomic mass units. Converting these values to grams per mole facilitates comparison with theoretical calculations and aids in compound identification.
Conclusion
The conversion from atomic mass units to grams per mole is a fundamental aspect of chemistry, bridging the gap between atomic and macroscopic scales. By understanding the theoretical foundations and practical implications of this conversion, chemists can navigate the complexities of stoichiometry, molecular weight determination, and material synthesis with confidence. As analytical techniques continue to advance, the precision and accuracy of these conversions will remain a cornerstone of chemical research and applications.



