Ch3oh Electron Geometry
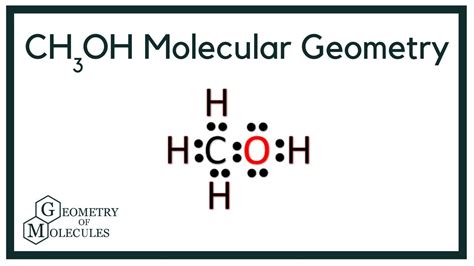
The electron geometry of CH3OH (methanol) is a fascinating topic that delves into the molecular structure and arrangement of electrons around the central atom. To understand this, let’s break down the concept of electron geometry and its application to methanol.
Electron Geometry Fundamentals
Electron geometry refers to the arrangement of electron pairs around a central atom in a molecule. This arrangement is determined by the principle of minimizing electron-electron repulsion, which is a fundamental concept in molecular geometry. The VSEPR (Valence Shell Electron Pair Repulsion) theory is commonly used to predict electron geometry.
According to VSEPR theory, electron pairs around a central atom will arrange themselves in a way that minimizes repulsion, resulting in a specific geometric shape. The number of electron pairs and their arrangement dictate the overall geometry of the molecule.
CH3OH Molecular Structure
Methanol (CH3OH) consists of a central carbon atom bonded to three hydrogen atoms and one hydroxyl group (-OH). The carbon atom has four valence electrons, which form bonds with the hydrogen and oxygen atoms. The oxygen atom in the hydroxyl group has six valence electrons, with two of these forming a bond with the carbon atom and the remaining four forming two lone pairs.
Determining Electron Geometry
To determine the electron geometry of CH3OH, we need to consider the electron pairs around the central carbon atom. The carbon atom has four electron pairs: three bonding pairs (with the hydrogen atoms) and one bonding pair with the oxygen atom. However, we must also consider the lone pairs on the oxygen atom, as they contribute to the overall electron repulsion.
Using the VSEPR theory, we can predict the electron geometry of CH3OH. The central carbon atom has four electron pairs, which would typically result in a tetrahedral electron geometry. However, the presence of the lone pairs on the oxygen atom affects the overall geometry.
Electron Geometry of CH3OH
The electron geometry of CH3OH is best described as a distorted tetrahedron or a trigonal pyramidal shape around the central carbon atom. The three bonding pairs (with the hydrogen atoms) and one bonding pair (with the oxygen atom) form a tetrahedral arrangement. However, the lone pairs on the oxygen atom cause a distortion in this geometry, resulting in a slightly compressed tetrahedron.
Molecular Geometry vs Electron Geometry
It’s essential to distinguish between molecular geometry and electron geometry. Molecular geometry refers to the arrangement of atoms in a molecule, whereas electron geometry considers the arrangement of electron pairs. In CH3OH, the molecular geometry around the central carbon atom is trigonal pyramidal, while the electron geometry is a distorted tetrahedron.
Factors Affecting Electron Geometry
Several factors can influence the electron geometry of a molecule, including:
- Lone Pairs: Lone pairs on surrounding atoms can cause distortions in the ideal electron geometry.
- Bonding Pairs: The number and arrangement of bonding pairs affect the overall electron geometry.
- Atomic Size: Larger atoms can cause distortions due to increased electron-electron repulsion.
- Formal Charge: The distribution of formal charges can impact electron geometry.
Practical Implications
Understanding the electron geometry of CH3OH is crucial in various fields, including chemistry, biochemistry, and materials science. The molecular structure and electron arrangement influence the physical and chemical properties of methanol, such as its reactivity, solubility, and intermolecular interactions.
For instance, the hydroxyl group (-OH) in methanol is responsible for its ability to form hydrogen bonds, which affects its boiling point, viscosity, and solubility in polar solvents. The electron geometry also plays a role in determining the molecule’s dipole moment, which is a measure of its polarity.
Comparative Analysis
Comparing the electron geometry of CH3OH with other molecules can provide valuable insights into the factors that influence molecular structure. For example:
| Molecule | Central Atom | Electron Geometry | Molecular Geometry |
|---|---|---|---|
| CH4 | Carbon | Tetrahedral | Tetrahedral |
| NH3 | Nitrogen | Tetrahedral | Trigonal Pyramidal |
| H2O | Oxygen | Tetrahedral | Bent |
| CH3OH | Carbon | Distorted Tetrahedral | Trigonal Pyramidal |

| Molecule | Central Atom | Electron Geometry | Molecular Geometry |
|---|---|---|---|
| CH4 | Carbon | Tetrahedral | Tetrahedral |
| NH3 | Nitrogen | Tetrahedral | Trigonal Pyramidal |
| H2O | Oxygen | Tetrahedral | Bent |
| CH3OH | Carbon | Distorted Tetrahedral | Trigonal Pyramidal |
This comparison highlights the impact of lone pairs and bonding pairs on electron geometry and molecular structure.
FAQ Section
What is the electron geometry of CH3OH?
+The electron geometry of CH3OH is a distorted tetrahedron or a trigonal pyramidal shape around the central carbon atom, due to the presence of lone pairs on the oxygen atom.
How does the hydroxyl group affect the electron geometry of CH3OH?
+The hydroxyl group (-OH) in CH3OH contributes to the overall electron repulsion, causing a distortion in the ideal tetrahedral electron geometry around the central carbon atom.
What is the difference between molecular geometry and electron geometry?
+Molecular geometry refers to the arrangement of atoms in a molecule, whereas electron geometry considers the arrangement of electron pairs around a central atom.
How does the electron geometry of CH3OH influence its physical properties?
+The electron geometry of CH3OH affects its physical properties, such as boiling point, viscosity, and solubility, through its influence on intermolecular interactions, including hydrogen bonding and dipole-dipole forces.
Can the electron geometry of CH3OH be predicted using VSEPR theory?
+Yes, the VSEPR (Valence Shell Electron Pair Repulsion) theory can be used to predict the electron geometry of CH3OH, taking into account the arrangement of bonding pairs and lone pairs around the central carbon atom.
Conclusion
In conclusion, the electron geometry of CH3OH is a distorted tetrahedron or a trigonal pyramidal shape around the central carbon atom, influenced by the presence of lone pairs on the oxygen atom. Understanding the electron geometry of methanol is essential for comprehending its molecular structure, physical properties, and chemical behavior. By applying the principles of VSEPR theory and considering the factors that affect electron geometry, we can gain valuable insights into the complex world of molecular structures and their implications in various fields.
As we’ve seen, the electron geometry of CH3OH is a fascinating topic that requires a deep understanding of molecular structure, electron arrangement, and the factors that influence them. By exploring this topic, we can develop a more nuanced appreciation for the complexity and elegance of chemical systems.


