Bohr Model For Fluorine
The Bohr model, proposed by Danish physicist Niels Bohr in 1913, revolutionized our understanding of atomic structure. While superseded by more advanced quantum mechanical models, it remains a valuable tool for visualizing electron arrangement in simpler atoms like fluorine. Let’s delve into the Bohr model for fluorine, exploring its structure, limitations, and significance. Fluorine’s Atomic Blueprint: A Nine-Electron System
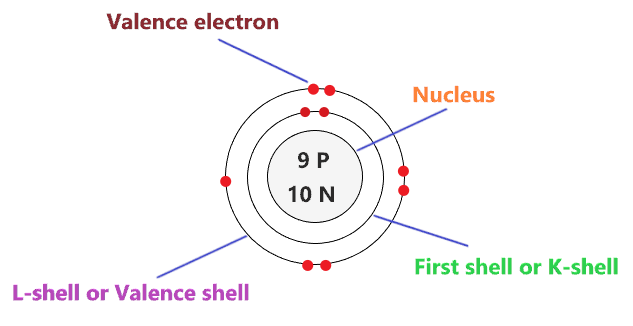
Fluorine, with atomic number 9, boasts nine protons in its nucleus and, in a neutral state, nine electrons orbiting it. The Bohr model simplifies this complexity by placing electrons in discrete energy levels, or shells, around the nucleus. Shell Structure and Electron Configuration
According to the Bohr model, fluorine’s nine electrons occupy the first two energy levels:
First Shell (K Shell): This innermost shell can hold a maximum of 2 electrons. Fluorine fills this shell completely.
Second Shell (L Shell): The second shell can accommodate up to 8 electrons. Fluorine places its remaining 7 electrons in this shell.
This electron configuration can be represented as: 1s² 2s² 2p⁵
Here, “1s²” indicates two electrons in the first shell’s s orbital, “2s²” signifies two electrons in the second shell’s s orbital, and “2p⁵” represents five electrons distributed among the three p orbitals of the second shell. Visualizing Fluorine’s Bohr Model
Imagine a small, dense nucleus at the center, representing the protons and neutrons. Surrounding this nucleus are two concentric circles, each representing an energy level. The first circle, closest to the nucleus, holds two electrons, while the second circle contains the remaining seven electrons.
Limitations of the Bohr Model
While the Bohr model offers a basic understanding of fluorine’s electron arrangement, it has significant limitations: * Ignores Electron Cloud: Electrons don’t orbit in neat circles like planets. They exist in probabilistic regions called orbitals, forming electron clouds. * Fails to Explain Spectral Lines: The model cannot fully explain the complex spectral lines observed in fluorine’s emission spectrum. * Doesn’t Account for Quantum Effects: It doesn’t incorporate principles like wave-particle duality and the uncertainty principle, fundamental to quantum mechanics.
Beyond Bohr: Quantum Mechanical Reality
The quantum mechanical model, which replaced the Bohr model, provides a more accurate description of fluorine’s electron behavior. It introduces concepts like:
- Orbitals: Regions in space where electrons are most likely to be found, described by mathematical functions.
- Quantum Numbers: Four numbers (n, l, mₗ, mₛ) that define the energy, shape, orientation, and spin of an electron within an atom.
- Electron Spin: Electrons possess an intrinsic angular momentum, or spin, which can be either “up” or “down.”
Significance of the Bohr Model for Fluorine
Despite its limitations, the Bohr model remains valuable for:
- Introductory Understanding: It provides a conceptual foundation for grasping basic atomic structure.
- Visual Representation: It offers a simple visual representation of electron arrangement, aiding in initial comprehension.
- Historical Context: It highlights the evolution of atomic theory and the transition from classical to quantum mechanics.
Fluorine’s Reactivity: A Consequence of Electron Configuration
Fluorine’s electron configuration, with seven electrons in its outermost shell, makes it highly reactive. It readily gains one electron to achieve a stable octet configuration, forming the fluoride ion (F⁻). This reactivity is why fluorine is the most electronegative element and a key component in many chemical compounds.
FAQ Section
Why does fluorine have a higher electronegativity than other halogens?
+ div>Fluorine's small atomic size and high effective nuclear charge result in a strong attraction for electrons, making it the most electronegative element.
How does the Bohr model differ from the quantum mechanical model for fluorine?
+The Bohr model uses circular orbits and discrete energy levels, while the quantum mechanical model employs orbitals and probability distributions, incorporating principles like wave-particle duality and electron spin.
What is the electron configuration of the fluoride ion (F⁻)?
+The fluoride ion has an electron configuration of 1s² 2s² 2p⁶, achieving a stable octet by gaining one electron.
Why is fluorine so reactive?
+Fluorine's high electronegativity and strong desire to complete its octet make it highly reactive, readily forming compounds with other elements.
What are some common compounds containing fluorine?
+Fluorine is found in compounds like sodium fluoride (NaF), calcium fluoride (CaF₂), and hydrofluoric acid (HF), which have diverse applications in dentistry, industry, and chemistry.
Conclusion: A Stepping Stone to Quantum Understanding
The Bohr model, while simplistic, serves as a crucial stepping stone in understanding fluorine’s atomic structure. It provides a foundational framework for grasping electron arrangement and highlights the need for more sophisticated quantum mechanical models. By appreciating both the strengths and limitations of the Bohr model, we gain a deeper understanding of the fascinating world of atoms and their behavior.


